Abstract
Background: Cytokine Storm (CS) is characterized by profound inflammation and multi-organ dysfunction. In a subset of COVID-19 patients with CS (COVID-CS), this has been shown to cause increased morbidity and mortality. There is, however, insufficient data to fully understand the precise role and scope of dysregulated cytokine responses in COVID-19 patients with hematological malignancies. We investigated the prognostic significance of pro-inflammatory cytokines with the overall outcome of patients with myeloid and lymphoid malignancies developing SARS-CoV-2 infection.
Methods: A retrospective study was conducted among 9,790 patients diagnosed or admitted with COVID-19 at MD Anderson Cancer Center between March 2020 and June 2022. The Palantir Foundry platform was used to collect and curate PHI via the D3CODE (Data Driven Determinants for COVID 19 Oncology Discovery Efforts) Initiative, through which baseline demographic, clinical, and biochemical characteristics of patients were collected. We used a combination of higher than laboratory cutoff values of C-reactive protein (CRP), interleukin 6 (IL-6) and ferritin measured within 14 days of COVID or malignancy as CS defining criteria. In order to distinguish CS due to COVID and CS due to underlying disease/treatment, two groups were identified as COVID-CS (C-CS+) and Non-COVID-CS (NC-CS+). The clinical features, diagnosis, and pathogenesis of C-CS+ were compared to those of NC-CS+ due to malignancy associated secondary hemophagocytic lymphohistiocytosis (sHLH) and chimeric antigen receptor (CAR-T) cell therapy associated Cytokine Release Syndrome (CRS). Finally, we compared these two cohorts with patients infected with SARS-CoV-2 without any clinical or laboratory evidence of CS, labelled as COVID without CS (CVD+CS-). The prognostic significance of these markers was examined in these three cohorts. Fit models and ROC curves were developed for comparing C-CS+, NC-CS+ and CVD+CS- groups. We recorded overall survival (OS) at last follow-up.
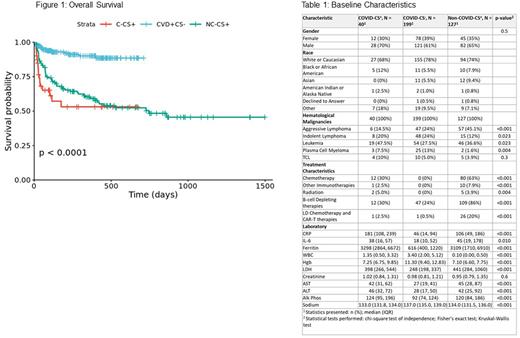
Results: We studied 366 patients in total: 40 were C-CS+, 127 were NC-CS+, and 199 were CVD+CS-. 113 patients had Leukemia (33%), 97 had Aggressive Lymphomas (30%), 71 had Indolent Lymphomas (19%), and 30 patients had Plasma Cell and Other Myelomas (8.2%). 168 patients (46%) received B-cell depleting therapy, 28 patients (8%) received CAR-T therapy, and 92 patients (25%) received chemotherapy. 36 patients (92%) in the C-CS+ group and 72 pts in the CVD+CS- (36%) and 105 pts (93%) in the NC-CS+ group were admitted. Length of stay (LOS) for the three groups C-CS+, CVD+CS-, NC-CS+ were 10 (R=4,20), 2 (R=0,5), 22 (R=11,30) (p<0.001). ICU admissions for the three groups C-CS+, CVD+CS-, NC-CS+ were 8 (21%), 4 (5.6%), 28 (25%) (p=0.002). Ferritin (p <0.001), IL-6 (p=0.01) and CRP (p <0.001) were higher for both CS+ groups vs. CS-, but had similar distributions between C-CS+ and NC-CS+. On univariate modeling, features with good predictive performance (AUC>8) included ferritin, albumin, Hgb, platelet count. ROC curve for multivariate model for all features had an AUC of 0.92. Multivariate analysis showed that CRP (OR-0.99, 95% CI 0.99-1, p=0.015), sodium (OR-1.13, 95% CI 1.02-1.26, p=0.022), and albumin (OR-3.04, 95% CI (1.41, 6.82), p=0.005) were associated with a higher mortality risk. Patients with C-CS+ had a lower OS compared to NC-CS+ and CVD+CS- (p=0.001). OS from both CS cohorts was better if they received prior vaccination (p=0.03). Finally, there was a striking OS in NC-CS+ patients when they received tocilizumab (p=0.005), while no difference in OS in C-CS+ in response to tocilizumab (p=0.81).
Conclusion: With the ongoing surge in COVID, patients with hematological malignancies are at higher risk particularly associated with CS. The results show that myeloid and lymphoid C-CS+ and NC-CS+ patients with higher levels of inflammatory markers are more likely to be admitted with ICU support and tend to have worse outcomes. Additionally, C-CS+ patients do not respond to cytokine targeted therapies. A consensus definition of COVID-CS may enable us to identify patients with CS who may benefit most from timely immune modulating therapies. Furthermore, lessons learned from both COVID and hematologic associated CS syndromes may provide insight into the pathologic immune dysregulation underlying CS, expedite rapid identification and implementation of therapeutic strategies.
Disclosures
Ahmed:Myeloid Therapeutics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Chimagen: Consultancy, Research Funding; Merck: Research Funding; Xencor: Research Funding. Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal